Medical Research Patient Safety: Funding Cuts Impact Efforts
Medical research patient safety is a fundamental concern that shapes the integrity and efficacy of clinical trials globally. As innovative therapies and treatments are explored, the oversight mechanisms such as Institutional Review Boards (IRBs) ensure that participant rights and well-being are prioritized throughout the research process. However, recent funding cuts have severely affected these efforts, risking ethical oversight in medical studies. Initiatives like the Harvard SMART IRB system play a crucial role in coordinating patient safety across multiple sites, but their functionality is threatened by financial setbacks. It is imperative for researchers, stakeholders, and policymakers to recognize the integral role of patient safety in research, particularly when examining the broader impacts of IRB oversight and funding policies on the future of medical advancements.
The realm of safeguarding participants in medical trials—often referred to as human research protection—encompasses vital ethical and regulatory frameworks that ensure individuals involved in studies are treated with respect and care. This includes the establishment of oversight bodies, such as Institutional Review Boards (IRBs), which critically evaluate research proposals and monitor participant safety throughout the study’s duration. Moreover, the recent cuts in funding not only jeopardize these ethical safeguards but also raise concerns about the integrity of the overall research landscape. The collaboration fostered by networks like the Harvard SMART IRB exemplifies how interconnectedness in research can enhance patient welfare systematically across various institutions. As we delve into this complex issue, it’s essential to understand the implications of funding decisions on the capacity to uphold participant safety in medical research.
The Importance of Patient Safety in Medical Research
Patient safety in medical research is paramount to the integrity and success of clinical studies. With human participants at the heart of medical research, ensuring their rights and welfare is not just ethical duty but a legal requirement. Institutions must obtain extensive oversight from Institutional Review Boards (IRBs) that rigorously assess each research proposal for potential risks and ethical considerations. These boards meticulously evaluate the informed consent process, ensuring participants are fully aware of what their involvement entails, including any possible risks and benefits.
In recent years, the emphasis on patient safety has intensified, particularly with the advent of new technologies and rapidly developing methodologies in clinical trials. Collaborative efforts across multiple sites demand robust systems, such as Harvard’s SMART IRB, to maintain high standards of oversight. This initiative not only aims to streamline the review process but also reinforces the commitment to safeguarding participants. As research becomes more collaborative and complex, safeguarding patient safety remains an indispensable component of ethical research practices.
IRB Oversight: Ensuring Ethical Compliance in Research
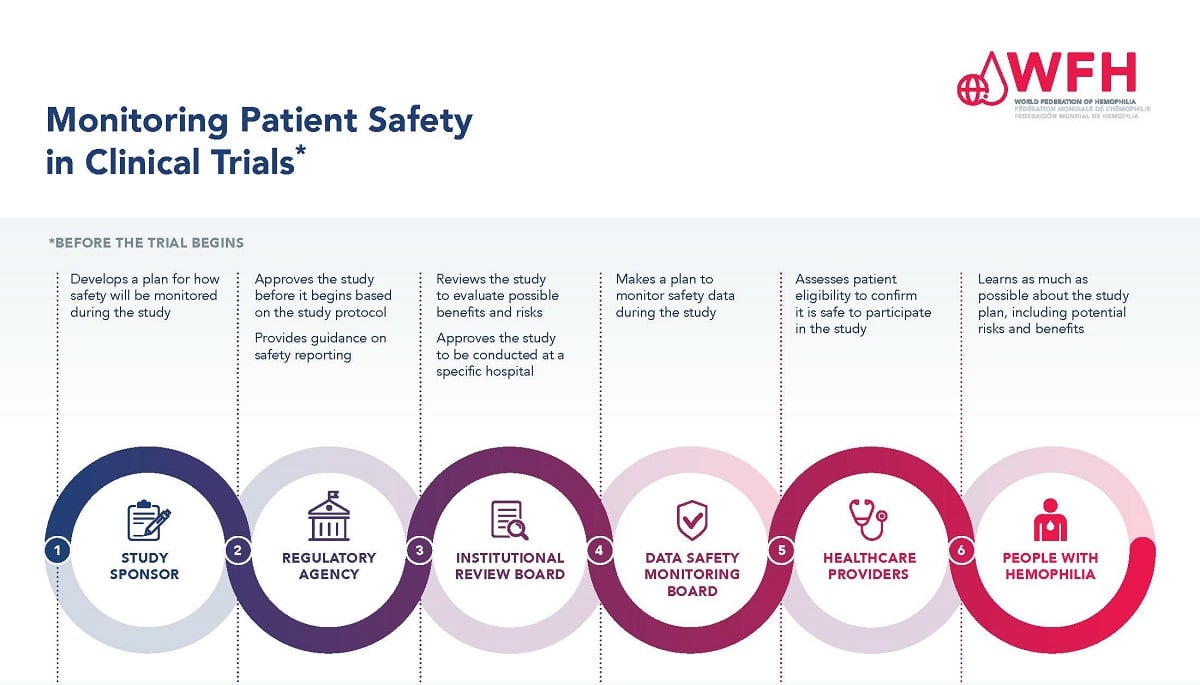
Institutional Review Boards (IRBs) form the backbone of ethical oversight in medical studies. Their role is crucial in reviewing research proposals for compliance with federal, local, and institutional regulations. IRBs are tasked with assessing risks to participants, which can significantly vary based on the nature of the study. They ensure that potential participants are not only informed of the risks involved in the research but also receive clear, transparent communication about the study’s objectives and potential benefits, establishing an informed consent framework that protects participants.
The requirement for a single IRB, especially in multi-site studies, has standardized the oversight process, minimizing administrative burdens while still prioritizing participant rights and safety. This centralized approach helps maintain ethical consistency across various research sites, making it easier to ensure that all participants receive the same level of protection and care. In an era where collaboration is key to advancing medical research, effective IRB oversight stands as a critical pillar supporting ethical compliance and participant safety.
The Impact of Funding Cuts on Patient Safety
Funding cuts in medical research can have severe implications for patient safety, particularly in terms of oversight and monitoring practices. When research grants and contracts are abruptly halted or reduced, it disrupts essential services provided by IRBs and support teams. Institutions may struggle to maintain rigorous safety standards, and ongoing studies might be forced to pause, placing participants at increased risk. An interruption in funding leads to fewer resources available for ethical training and oversight, which are critical to ensuring the welfare of research subjects.
For instance, the recent federal funding cuts to Harvard have had immediate repercussions on studies utilizing the SMART IRB system. With ongoing projects facing stop-work orders, the safety and rights of participants are jeopardized. These disruptions can lead not only to delays in obtaining results but also to potential harm for participants who cannot be monitored or supported adequately. This highlights the systemic relationship between robust funding and the protective measures vital for fostering a safe research environment.
Collaborative Research Models: Balancing Efficiency and Ethics
Collaborative research models, such as those facilitated by the SMART IRB initiative, demonstrate the importance of balancing efficiency with ethical oversight in medical studies. By allowing multiple institutions to share a common review process, these models aim to streamline research efforts while maintaining rigorous ethical standards. Such collaboration is essential, especially for large-scale studies where various sites contribute to the data pool. However, these efficiencies must not undermine patient safety or ethical responsibilities.
The effectiveness of collaborative models hinges on unwavering commitment from participating institutions to adhere to ethical guidelines and prioritize patient welfare. Each institution involved must uphold the principles set forth by the IRB while fostering communication about the research process with participants. By ensuring a transparent and ethical approach, researchers can enhance trust with participants, ultimately leading to more robust study outcomes and a renewed faith in the medical research process.
The Role of Ethical Oversight in Building Public Trust
Ethical oversight is integral to building and maintaining public trust in medical research. Historical events—such as the Tuskegee Syphilis Study—have left lasting scars on public perception, illustrating the fundamental necessity of trustworthiness and ethical conduct in research. Today, IRBs function as guardians of participant rights, ensuring that research is conducted transparently and ethically. Participation in research requires not only informed consent but also the assurance that ethical standards are upheld diligently throughout the study.
By actively engaging with communities and prioritizing participants’ rights, researchers can foster a culture of trust and collaboration. This proactive approach involves communicating outcomes, risks, and ethical practices transparently. Without such engagement, skepticism and mistrust towards research can flourish, jeopardizing future studies and ultimately hindering advancements in medical science. Thus, ethical oversight is essential not only for immediate purposes but also for the long-term viability of research initiatives.
The Importance of Informed Consent in Research Ethics
Informed consent stands as a cornerstone of ethical research practices, encompassing not just legal requirements but also moral obligations toward participants. It ensures that individuals understand the nature of the research, including potential risks and benefits, before agreeing to participate. Clear communication during this process is essential, as it allows participants to make well-informed decisions regarding their involvement in medical studies. Without proper informed consent procedures, participants could be exposed to unnecessary risks, drastically undermining patient safety.
Furthermore, the process of obtaining informed consent should not be viewed as a one-time event but rather as an ongoing dialogue between researchers and participants. Researchers must be prepared to address questions and concerns as they arise throughout the study’s duration, reinforcing trust and ensuring participants feel valued and protected. As research methods evolve, maintaining a strong focus on informed consent remains crucial, as it directly impacts the integrity of the research and the safety of all participants involved.
Navigating the Challenges of Multisite Research
Multisite research presents unique challenges pertaining to patient safety and regulatory compliance. These studies involve collaboration across various sites, which can complicate oversight and coordination. The role of a single IRB becomes particularly important in these scenarios, as it streamlines the review process and centralizes oversight. This efficiency helps mitigate delays, enabling researchers to focus on the ethical aspects of their studies rather than getting bogged down in administrative hurdles.
However, the complexity of multisite studies necessitates that all participating institutions remain vigilant in adhering to ethical protocols established by the IRB. Each site must ensure that the rights and safety of participants are consistently prioritized, even when researchers are working across different locations. Challenges such as differing state regulations or institutional policies must be navigated carefully to maintain compliance and safeguard participant welfare. Only through diligent adherence to ethical standards can multisite research fully realize its potential to contribute to significant advancements in patient care.
The Future of Ethical Standards in Medical Research
As funding landscapes shift, so too must the frameworks that preserve ethical standards in medical research. Emerging technologies and methodologies present both opportunities and challenges, necessitating continual evolution of IRB practices and oversight processes. In the wake of recent funding cuts and the evolving nature of research collaboration, institutions must remain adaptable while ensuring that patient safety and ethical integrity are never compromised. This adaptability is crucial in fostering innovation while simultaneously respecting established ethical boundaries.
Looking ahead, it is imperative for researchers, administrators, and regulatory bodies to engage in ongoing dialogue about ethical standards. Prioritizing advancements in training and resources for IRBs will empower them to navigate the complexities of modern research environments effectively. By maintaining a strong focus on ethics and collaborative efforts, the research community can better safeguard participants while still pushing the boundaries of scientific exploration.
Addressing Public Skepticism in Clinical Trials
Public skepticism surrounding clinical trials poses a significant challenge for researchers. Historical abuses and ethical failings in medical research have led many to question the intentions and safety of clinical trials. As trust in the research community diminishes, it becomes essential for institutions to engage openly with the public about the safeguards in place to protect research participants, including the roles of IRBs in ensuring ethical oversight.
Researchers must be proactive in addressing concerns within the community, highlighting the rigorous review processes that studies undergo before initiation. By fostering transparency and open communication, the research community can work to rebuild trust and encourage greater participation in clinical trials. This effort is vital not only for the health of individual patients but also for the advancement of medical knowledge and treatment options available to the public.
The Global Impact of Research Ethics
In an era marked by globalization, the approach to research ethics has broader implications that transcend borders. Ethical standards in medical research are becoming increasingly important as researchers engage in international collaborations. Different countries have varying regulations and ethical considerations, necessitating a comprehensive understanding of diverse cultural perspectives on patient safety and informed consent. Thus, fostering an international dialogue around ethics in research can enhance global approaches to protecting participants.
Moreover, as the reach of clinical trials expands globally, it is critical for ethical guidelines to remain robust yet flexible enough to accommodate different cultural contexts. The cross-border nature of research can lead to disparities in how participant rights are protected, so establishing best practices that integrate local values while ensuring the fundamental principles of patient safety are maintained is essential. Collaboration among international bodies can help harmonize these efforts, ultimately contributing to a more ethically sound research environment worldwide.
Frequently Asked Questions
What is the significance of patient safety in research projects?
Patient safety in research is critical as it ensures that participants’ rights and welfare are protected throughout medical studies. Institutional Review Boards (IRBs) are responsible for overseeing research protocols to safeguard participants from potential risks, thereby enhancing the ethical conduct of medical research.
How does IRB oversight contribute to patient safety in medical research?
IRB oversight plays a fundamental role in ensuring patient safety in medical research by rigorously reviewing study proposals for ethical compliance, assessing risk-to-benefit ratios, and continuously monitoring participant protection throughout the study lifecycle. This oversight helps maintain high standards of care and ethical integrity in research involving human subjects.
What impact do funding cuts have on patient safety in medical research?
Funding cuts significantly hinder patient safety in medical research by disrupting the operations of IRBs and compromising their ability to oversee studies effectively. A halt in resources may lead to delays in research, increased risks for participants, and diminished trust in the research process, ultimately jeopardizing the health and safety of research subjects.
How does Harvard SMART IRB enhance patient safety in collaborative research?
The Harvard SMART IRB provides a unified framework for ethical oversight, streamlining the review process for multi-site studies. By consolidating IRB functions, it ensures efficient monitoring of patient safety across various institutions, thereby promoting high-quality standards in research and facilitating quicker access to potentially beneficial treatments for participants.
What ethical principles guide patient safety in medical studies?
Patient safety in medical studies is governed by key ethical principles such as respect for persons (informed consent), beneficence (maximizing benefits while minimizing harm), and justice (fair treatment in participant selection). These principles guide IRBs in their mission to oversee research and protect the rights and welfare of human subjects engaged in medical research.
In what ways do IRBs address the historical lessons about patient safety in research?
IRBs were established in response to historical unethical practices in medical research, such as the Tuskegee syphilis study and the Willowbrook hepatitis studies. By enforcing guidelines and ethical standards, IRBs aim to prevent such violations, ensuring that every research participant’s safety and trust are prioritized in modern medical studies.
What role do researchers play in ensuring patient safety during medical trials?
Researchers play a pivotal role in ensuring patient safety by designing studies that prioritize participant welfare, seeking IRB approval, and adhering to protocols that safeguard against risks. They must also maintain transparent communication, regularly monitor participant health, and report any adverse events to uphold the integrity of their research.
How does public trust relate to patient safety in ongoing medical research?
Public trust is essential to patient safety in medical research, as skepticism can lead to decreased participant enrollment and reluctance to engage in studies. Institutions must foster transparency, address ethical concerns, and demonstrate commitment to patient safety to maintain confidence and encourage community participation in vital research initiatives.
What resources are available for participants concerned about their safety in medical research?
Participants concerned about their safety in medical research can rely on IRBs as a resource for questions and support. IRBs provide oversight, ensuring that participants understand the research risks and benefits, and they serve as a point of contact for any ethical concerns regarding study involvement.
How can collaboration improve patient safety in medical research?
Collaboration among multiple research institutions enhances patient safety in medical research by sharing best practices, pooling resources, and ensuring comprehensive oversight. Collaborative efforts, facilitated by structures like the SMART IRB, can lead to more efficient studies that prioritize participant welfare and ethical standards.
| Key Points | Details |
|---|---|
| Impact of Funding Cuts | Over $2 billion in federal research funding cuts disrupt medical research oversight and patient safety. |
| Role of IRBs | Institutional Review Boards (IRBs) safeguard participant rights and ensure compliance with laws and regulations. |
| Importance of Oversight | IRBs serve as checks and balances in research, preventing harm to participants and ensuring ethical conduct. |
| Historical Context | IRBs were established to prevent ethical violations in research, notably after the Tuskegee Study and other historical abuses. |
| Consequences of Research Delays | Funding cuts lead to halted studies and increased mistrust in medical research, ultimately endangering patient safety. |
| Continued Commitment | Despite funding challenges, efforts to maintain patient safety in research continue, supported by Harvard Medical School. |
Summary
Medical research patient safety is a critical concern that has been adversely affected by recent funding cuts. The disruption of federal grants has undermined the systems in place to protect patients involved in clinical trials. Institutional Review Boards (IRBs) play an essential role in ensuring ethical oversight and compliance, safeguarding the rights and welfare of research participants. Funding reductions threaten the ability of IRBs to operate effectively, risking the safety of patients and the integrity of medical research. Moving forward, it is imperative to address these funding issues to uphold the highest standards of patient safety and trust in the research community.